Which Is the Best Summary of the Kinetic Theory
What does a high boiling. Kinetic theory is the atomistic description of gases as well as liquids and solids.
The Kinetic Molecular Theory Of Gases Fiveable
Friction generates more kinetic energy.
. 2499 year tax. It is also known as kinetic molecular theory or the collision theory. Kinetic theory of gases.
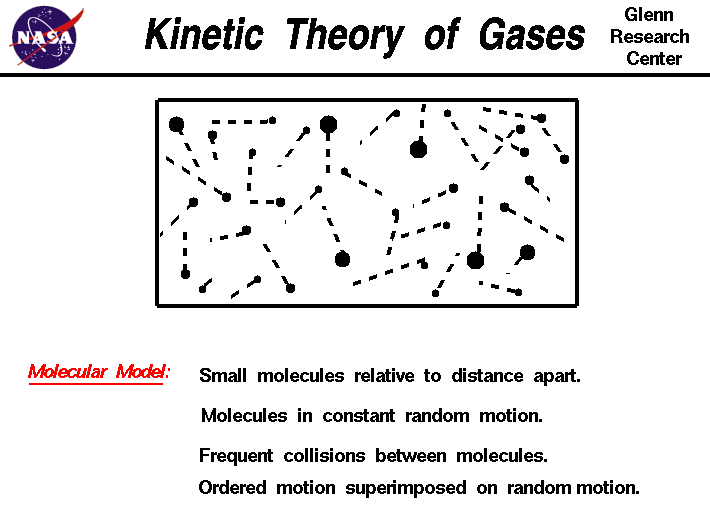
The Kinetic-Molecular Theory Explains the Behavior of Gases Part II According to Grahams law the molecules of a gas are in rapid motion and the molecules themselves are small. Kinetic Theory of Matter. Molecules are very small relative to the distances between them.
Note that this final topic has very little pedagogically to do with all the stuff weve been talking about all quarter. The kinetic theory of matter states that all matter is made of small particles that are in random motion and that have space between them. The kinetic theory of gases was developed by Daniel Bernoulli 17001782 who is best known in physics for his work on fluid flow hydrodynamics.
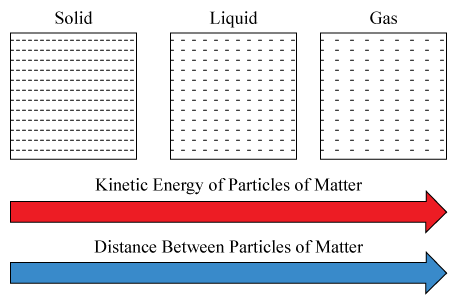
The average distance between the molecules of a gas is large compared to the size of the molecules. The Kinetic Theory of Matter Lesson Summary The Four Phases of Matter Matter is anything that has mass and volume. Assumptions of Kinetic Theory of Gases 1.
Matter that does not have a definite shape or volume. Potential energy is converted to kinetic energy. Kinetic energy is only present where there is motion in an object.
That is matter is anything that requires energy to accelerate or change its. Energy cannot be created or destroyed. From a general summary to chapter summaries to explanations of famous quotes the SparkNotes Kinetic Molecular Theory Study Guide has everything you need to ace quizzes tests and essays.
Is the fourth state of matter distinct from the other states. Ii The total volume of molecules is negligible in comparison to the total volume occupied by the gas. In other words the energy of an ideal gas is entirely kinetic.
Collisions between molecules are perfectly elastic. An ideal gas has following characteristics. The average distance a molecule travels.
Kinetic Theory states that pressure is not cause by molecule that push molecule to each other and pressure is caused by molecule colliding with the each other and with the container. Atoms and molecules are always in motion. All collision between particles are perfectly elastic.
The size of a molecule is negligible as compared with the average distance between two molecules. Kinetic theory explains the behaviour of gases based on the idea that the gas consists of rapidly moving atoms or molecules. I There are no interactions between molecules.
Early classical mechanics as propounded by Isaac Newton especially that based on his laws of motion and theory of gravity. 1 -energy given out by particles of liquid -particles lose KE and begin moving more slowly 2 -particles no longer have enough energy to move abt freely when temp is low enough -some particles start settling into fixed positions 3 -all particles settle into fixed positions -particles only vibrate about their fixed positions. This means that no matter what phase matter is in it is.
There are five principles to the kinetic theory. So for better or for worse the topic of transport will stand as its own independent module. These small particles are in constant motion.
During the random motion the molecules collide with one another and with the wall of the vessel. All gases consist of molecules which are rigid elastic spheres identical in all respect for a given gas. The molecules of a gas are in constant and random motion The temperature of a gas depends on its average kinetic energy avg 12mv2 32kT.
There are no attractive forces between the particles or their. Matter is composed of tiny particles. Kinetic theory of gases is built on two principles.
After a long foray into sophisticated field theories were going to jump into a ball-pit and play with transport. All matter is made up of tiny particles that are in constant motion. All matter is composed of small particles.
The amazing thing about the kinetic molecular theory is that it can be used to derive the ideal gas law. These are in constant motion. Bernoullis work predates the atomistic view of matter established by Dalton.
The kinetic theory of gases describes a gas as a large number of small particles atoms or molecules all of which are in constant random motion. Ideal Gas An ideal gas or a perfect gas is that gas which strictly obeys gas laws such as Boyles law Charles law Gay Lussacs law etc. A fluid state of matter having no.
The basic assumption of kinetic theory is that the measurable properties of gases liquids and solids reflect the combined actions of countless numbers of atoms and molecules. They do not lose energy in collision with each other or the walls of their container. Kinetic theory In physics a theory dealing with matter in terms of the forces between particles and the energies they possess.
Kinetic theory explain you property of gas such as 1 pressure 2 temperature 3 volume. Gas molecules are point-like objects that experience only elastic collisions. Atoms and molecules are always in motion.
The best summary of kinetic energy is energy of motion. What is the best summary of the kinetic theory. Basic assumptions of the kinetic theory.
Molecules are in constant random chaotic motion. Which is the best summary of the kinetic theory. Kinetic Theory.
For example the pressure exerted on the walls of a bicycle tire is produced by the impacts of an enormous number of air molecules. Atomic motion is constant at all temperatures. Is sometimes called the molecular kinetic theory MKT Postulates All matter is composed of particles molecules in general but also atoms ions and free electrons.

The Kinetic Molecular Theory Of Matter Introduction To Chemistry

Kinetic Theory Of Gases Explanation Assumptions Postulates Formula
Comments
Post a Comment